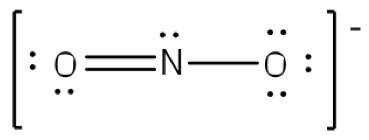Fh Lewis Structure

The lewis structure for li is li with one dot to the right of the element.
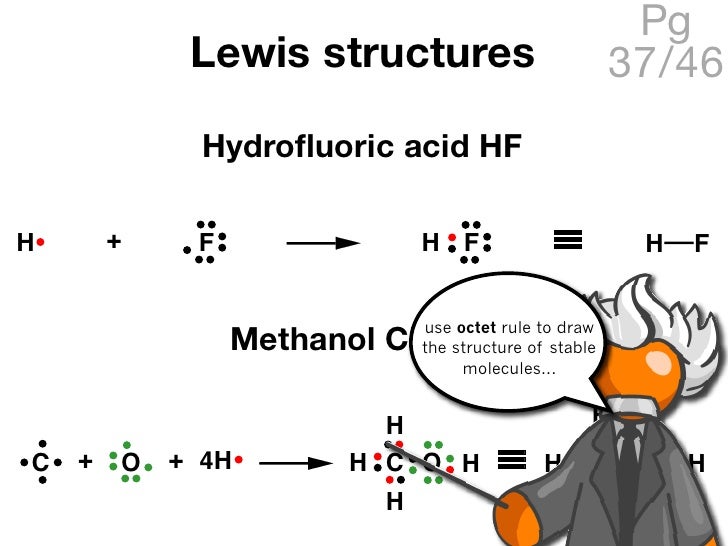
Fh lewis structure. What is the lewis structure of asf5. I quickly take you through how to draw the lewis structure of hf hydrogen fluoride. Lewis structures are not generally expected to represent the geometrical shape of a molecule one reason being that they must be drawn in a plane while the molecule itself may not be planar. Lewis structure was created in 1916.
I think it s similar to the lewis structure for pcl5. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist. A step by step explanation of how to write the hf lewis structure hydrofluoric acid first we count the valence electrons for hf using the periodic table. So if you type that structure into google you should receive the.
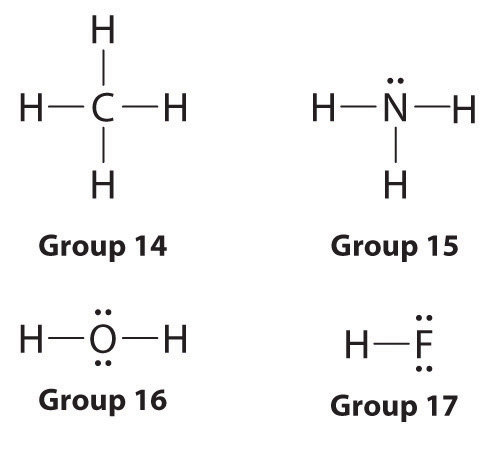
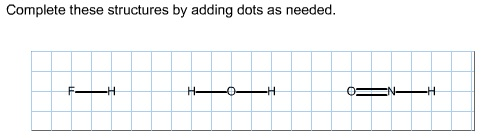
With the lewis structure for hf remember that hydrogen only needs 2 valence electrons to have a full outer shell. I also go over the shape and bond angle. Be sure that you don t use more than the 8 valence electrons available. A lewis structure is expected to show a which atoms in the molecule are bonded together and.
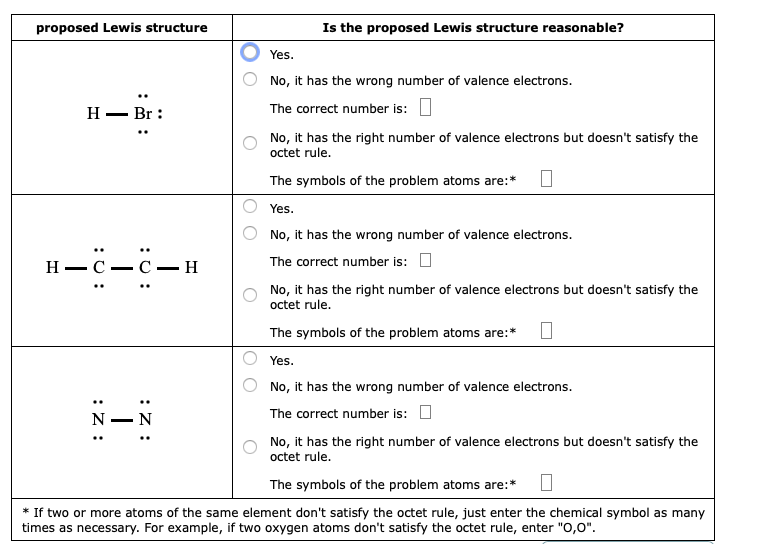
Both lewis structures have a net formal charge of zero but the structure on the right has a 1 charge on the more electronegative atom o.